Marine Tropical Aquarium

Marine aquariums are certainly more time demanding and difficult to set-up than freshwater aquariums. Marine aquariums require much more careful treatment and a more in-depth knowledge of chemistry and biology than freshwater aquariums.
Accessories, animals and aquarium prices, which must be considerably larger then the ones used for freshwater aquariums, certainly affect the aquarist who intends to move from freshwater to marine.
I explicitly said the phrase "intends to move from freshwater to marine" because it is my belief that a good "training" of at least one or two years by the aquarist in a freshwater aquarium will avoid disappointments and waste of money in the management of a marine aquarium.
On the other hand I have seen stubborn boys that want at all costs to set up a marine as a first aquarium and by following the advice of experienced aquarists succeed to keep an optimal balance in the aquarium. Passion is the host!
In this Neperos article we will talk deep about the issues concerning the marine aquarium, both with articles for the novice and for the expert.
Logically we will leave aside all those notions that assume that those who enter the marine world and those who already live in this world already have their personal baggage; I'm talking about accessories, chemistry and biology knowledges on both freshwater and marine aquariums.
Our journey will start from the most different element from fresh water:

Salt water from the sea
Water is one of the most complex elements that must be known by the aquarist, because it is in it that our friends live, move, reproduce.
As a chemical element, water has the formula H2O, which indicates that in the molecules that compose water there are two atoms of Hydrogen and one of Oxygen.
The characteristics of the water that affect the aquarist are numerous, but not difficult to understand:
1) PH, hydrogenetic concentration of water, has a direct effect on all living organisms in water and is responsible for the development of all their vital processes. In the tropical marine aquarium must remain between 8.2 and 8.4.
2) KH or carbonate hardness that indicates the concentration of carbonates of calcium and magnesium carbonates, very important because they are absorbed by all the aquatic organisms, in particular the corals which absorb calcium and magnesium in order to growth.
In the tropical marine aquarium it must remain at 12 ° or 14 °.
3) Of great importance is the concentration of oxygen, which must be kept saturated for various reasons: the most important is the reduction by the nitrifying bacteria of organic substances.
4) Salinity: measures the amount of salts dissolved in water and it is measured in parts per thousand, ie ‰, for example, a salinity of 38 ‰ indicates that in a liter of water 338 grams of salt are dissolved.
The salinity of a tropical marine aquarium is about 33 \ 34 ‰, ie 33 \ 34 grams of salt dissolved in water.
The densimeter, for its part, will measure the density of water, which must be between 1020 and 1023 at a temperature of 25 °C, but this accessory will be presented later.

Synthetic salts for the marine aquarium
It is evident that the natural sea water contains more than one salt: in it is dissolved a complex mixture of saline composed, in different percentages, each composed by an enormous number of elements.
Although the salinity may vary in different marine areas, it has been demonstrated that the composition of sea water is strictly constant, with the exception of small coastal areas where water courses rich in particular salts are poured into the sea.
The compounds best represented in marine waters are sodium and magnesium chloride, magnesium sulfate, calcium and potassium, potassium bromide, calcium carbonate.
Other "ninety" compounds (about) do not exceed the concentration of 0.2 mg / l, among which we may also find gold, titanium, vanadium, nickel and many other noble metals, but ... stay calm, to become rich you should drain a gulf, but I do not know if it would still be enough !!
The synthetic waters currently available on the market are generally prepared with very complex processes and are able to simulate most of the compounds of natural sea water, therefore, also considering the pollution level reached by our seas today, I highly recommend using synthetic water, even if live in a stilt house on the sea!
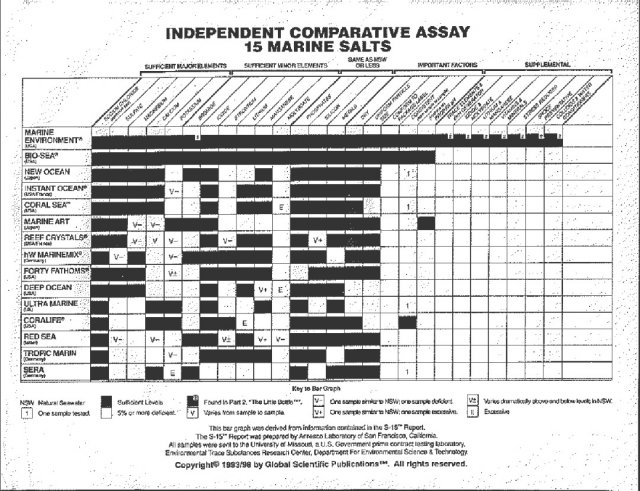
In any case, with regard to synthetic salts, we must be careful. Low quality salts do not fully meet the demands of our guests in the aquarium. The water, as already mentioned, is the most important component for our fishes. A table displaying an information sheet on the main salts available on the market is also available. The comparative table is from the Laboratory of San Francisco, California.
The oligoelements_/
From the Greek "Oligos" = little.
Elements not presented in marine waters.
As the etymology of the term suggests, these are elements present in minimal quantities in sea water, sometimes so rare that they can not be identified by means of normal analytical systems.
Yet, in spite of the modest quantities in which they are present, these elements often play a fundamental role in life in the seas and many organisms are sensitive to the presence of these elements.
This can not be surprising if we take into account that many organisms are able to accumulate these substances in their internal liquids, then using them for fundamental vital processes.
This is the case of the Ascidians and of numerous other Tunicates, which succeed in accumulating Vanadium up to concentrations 20,000 times higher than the concentration in which this element is present in sea water.
All fish suffer, in the absence of trace elements. They present in the beginning particularly faded colors and, subsequently, epidermal degeneration, goiter formation and functional imbalances of various types.
Oligoelements or trace elements must be integrated periodically and without ever "forgetting", because they are absorbed by the animals at the speed of light!
In this case too, the use of high quality salts will greatly help to maintain ideal concentrations of trace elements in the aquarium.
Suffice it to say that there are salts that contain the second component, which is nothing more than liquid or powder trace elements depending on the commercial brand.
Certainly their price is considerably higher, but also the result on fish and invertebrates will be far above the average !!