PiHKAL: The Chemical Story 13

eZine lover (@eZine)
Published in
PiHKAL
· 19 Jul 2023
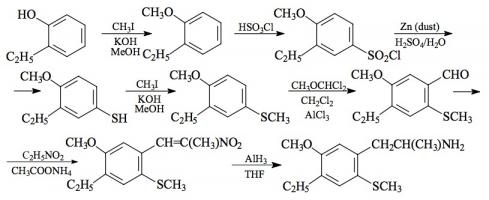
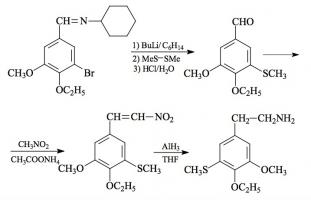
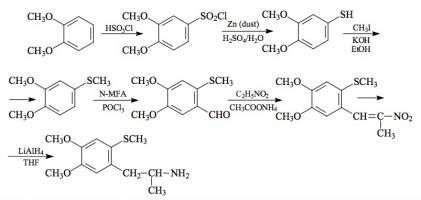
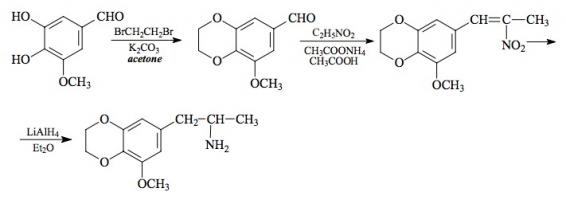
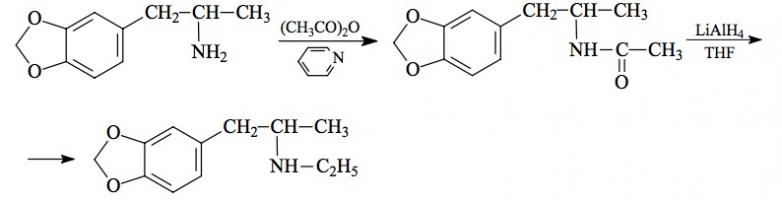
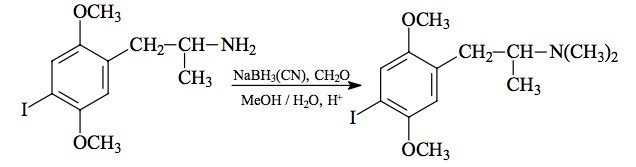
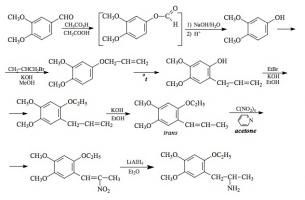
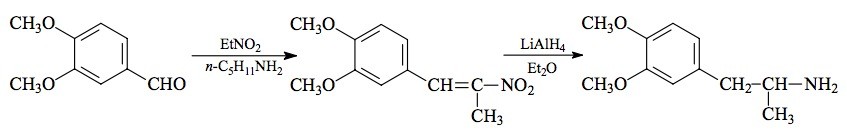
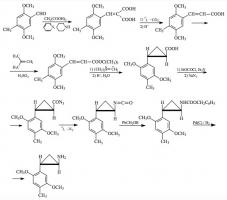
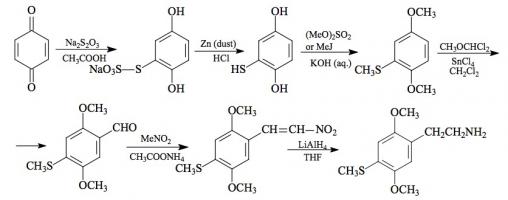
#169 2-TOET; 4-ETHYL-5-METHOXY-2-METHYLTHIOAMPHETAMINE SYNTHESIS: A mixture of 24.4 g ortho-ethylphenol and 18.9 mL methyl iodide was added to a solution of 15.6 g 85% KOH in 100 mL hot MeOH. The mixture was kept at reflux temperature overnight, stripped as much as possible of the MeOH, and poured into 1 L H2O. An excess of 5% NaOH was added and this was extracted with 3x75 mL CH2Cl2. The pooled extracts were washed with 1% NaOH, and the solvent removed under vacuum to give 32.8 g of a pale amber oil. This was distilled at 55-65 °C at 0.4 mm/Hg to yield 22.0 g of 2-ethylanisole as a colorless oil. To a 21.7 g sample of 2-ethylanisole...